Background: Exportin 1 (XPO1) is involved in the nuclear export of tumor suppressor proteins (TSPs) and associated with poor prognosis and drug resistance in multiple myeloma. Selinexor is a novel, oral, first-in-class selective inhibitor of XPO1 that was approved by the US FDA for the treatment of patients with relapsed/ refractory multiple myeloma (RRMM) based on the STORM study of selinexor (80mg biweekly) in combination with dexamethasone (20mg biweekly) (Xd). Furthermore, in pre-clinical studies, selinexor played a synergistic anti-tumor role with chemotherapy drugs, however clinical data is lacking on such combination treatment.
Methods: The LAUNCH study is a multicenter, open-label study (NCT04877275) which enrolled RRMM patients at least one prior treatment. There are two arms in this study, with 25 patients in each arm. XDd Arm: Selinexor 80mg d1,8,15,22, Dexamethasone 40mg d1,8,15,22, and Liposomal Doxorubicin 25-35 mg/m 2 d1; XCd Arm: Selinexor 100mg d1,8,15,22, Dexamethasone 40mg d1,8,15,22, and Cyclophosphamide 300mg/m2 d1,8,15,22. 28d per cycle in both arms. The primary endpoint is objective response rate (ORR), secondary endpoints including clinical benefit rate (CBR), duration of response (DOR) and 1-year progression free survival (PFS)
Results: As of July 26, 2023, 44 patients were enrolled (25 in XDd and 19 in XCd), XDd arm has completed recruitment. Of these, 23 patients were male and 21 were female. The median age was 60 years (range: 29-74years). The median time since initial diagnosis was 4.0 years (range: 0.5-15.5years). The median number of prior treatments was 3 (range: 1- 10). There were 13 patients (29.5%) with high-risk cytogenetic abnormalities, 8 patients (18.2%) with extramedullary disease (EMD).
Eight patients (18.2%) received prior autologous stem cell transplantation and 7 patients (15.9%) received CAR-T therapy. Forty-two patients (95.5%) were exposed to bortezomib, 35 patients (79.5%) were exposed to lenalidomide, 18 patients (40.9%) exposed to pomalidomide, and 10 patients (22.7%) exposed to daratumumab.
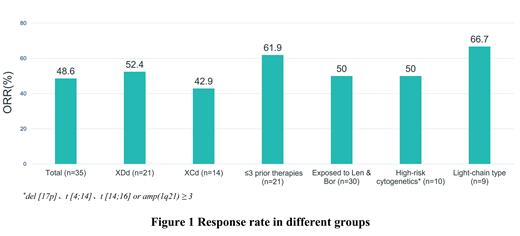
The median treatment duration of all patients was 13 weeks (range: 1- 48). Thirteen patients remained on treatment. Based on the 35 patients evaluated for efficacy, the ORR was 48.6% (52.4% in XDd arm, n=21; and 42.9% in XCd arm, n=14) including 1 CR, 1 VGPR and 15 PRs. Three patients achieved MR and 12 patients had stable disease.
The most common hematological adverse events (AEs) (All Grades, Grade ≥3) were thrombocytopenia (61.4%, 27.3%), leukopenia (54.5%, 34.1%), and anemia (45.5%, 29.5%). The most common non-hematological AEs (All Grades, Grade ≥3) were fatigue (47.7%, 2.3%) nausea (45.5%, 2.3%) and vomiting (31.8%, 4.5%). Most treatment related adverse events occurred in the first 8 weeks and were alleviated over time.
Conclusion: Once weekly selinexor and dexamethasone (Xd) combined with cyclophosphamide or liposome doxorubicin showed encouraging efficacy and manageable safety in treatment for RRMM. This enrollment is still ongoing and data on all participants will be reported in due course.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal